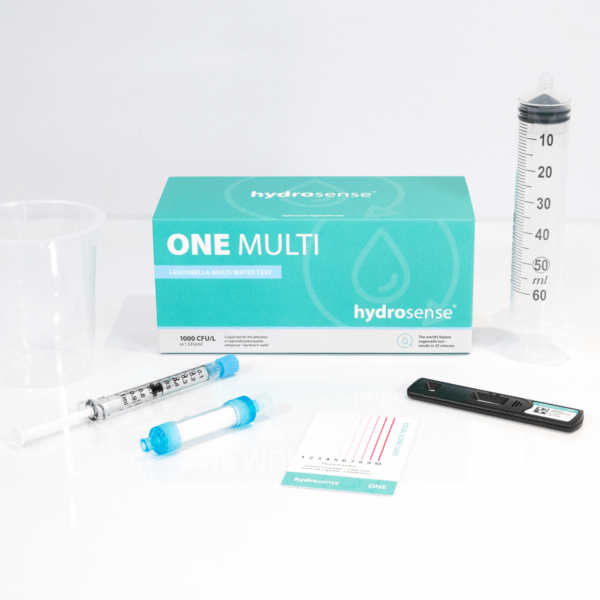
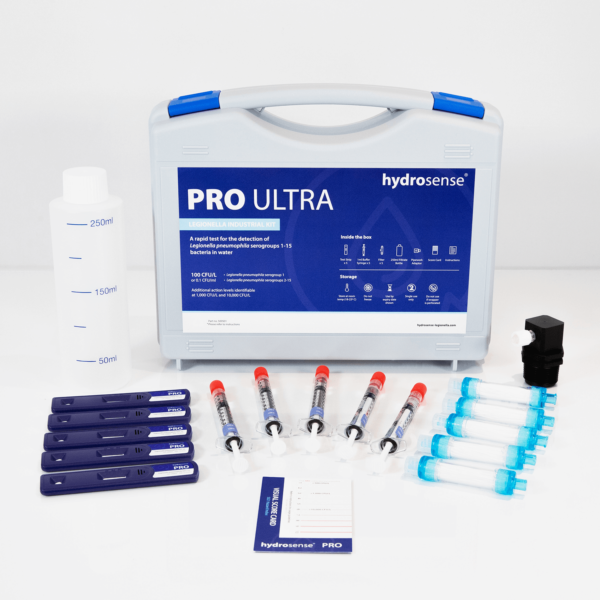
General FAQ
The Hydrosense range of tests are specific to the species Legionella pneumophila. The ONE range of tests detects L. pneumophila sg1, whilst the PRO range detects both L. pneumophila sg1 and L. pneumophila sg2-15. Legionella pneumophila serogroups 1-15 together are responsible for around 97% 1 of all global cases of Legionnaries’ disease with sg1 accounting for 90% 2 of the cases and the remainder caused by sg2-15. Other species of Legionella are much less common at causing human disease and therefore are not considered such high risk organisms.
The Hydrosense tests have all been rigorously tested and validated in house at Hydrosense to ensure the tests work exactly as specified. In addition, the Hydrosense test methods, both for ONE and PRO variations, have been independently validated by a UKAS accredited laboratory in the UK to verify the tests ability to detect Legionella pneumophila (sg1 and/or sg2-15) at the detection levels stated in our claims.
Both the Hydrosense ONE and PRO tests have also been validated by a CDC ELITE laboratory in the USA and the Hydrosense PRO test has been evaluated against lab culture using the CDC ELITE proficiency testing scheme and was found to be 100% accurate at identifying L. pneumophila from the blind samples used in testing scheme.
Many products sold in the EU carry a CE mark which indicates that the product is in compliance with European Directives. At present, there is no EU directive on any Legionella water tests. As a result, CE marking is not applicable.
UKAS Accreditation is only available to laboratories and therefore UKAS accreditation is not applicable to the Hydrosense tests. It is possible that a UKAS accredited lab could carry out Hydrosense Legionella rapid tests, but the tests are generally designed for on-site, rather than lab based testing.
MSDS are available for all of our Hydrosense test kits. To request an MSDS contact a member of the team at
Yes. Every batch of Hydrosense tests produced undergoes stringent QC testing to ensure it meets our specifications. A certificate of conformance can be obtained by contacting
Yes. The Hydrosense range of Legionella tests are highly efficient for testing multiple samples at one time and takes only 25 minutes to provide results. The filtration process typically takes well under 5 minutes. Several samples can be collected, and multiple different tests can be performed simultaneously.
Lab culture methods for Legionella testing, though widely recommended, have significant limitations. The ISO standard (11731:2017) indicates that lab culture recovery rates are often lower than 64%, meaning up to 35% of tests may return false negatives. Furthermore, lab culture fails to detect Viable but Non-Culturable (VBNC) forms of Legionella, which still pose significant infection risks.
Relying on lab culture alone may delay crucial action since results take up to two weeks — during this wait, people have been exposed to potential increased infection risks. Hydrosense, by contrast, delivers results in just 25 minutes. While lab tests are necessary for compliance in some cases, supplementing them with additional testing methods like Hydrosense can offer more immediate and reliable protection.
Legionnaires’ disease presents a significant risk to public health and as such, in many industries, the control of Legionella bacteria is carefully regulated by specific health and safety laws.
However, some businesses and sectors have a much higher risk when it comes to Legionella and Legionnaires’ disease than others. The main risk areas that encourage the rapid growth and spread of Legionella include cooling towers, potable water systems, showers, sinks, spas/hot tubs, swimming pools, water tanks, car washes, decorative fountains and others. Read more about risk factors in different sectors here.
Yes, The Hydrosense Legionella Tests have been developed to be very easy to conduct and with the help of the instructions included in the kit, and instructional videos available online, anyone can perform the test.
The Hydrosense test kits are particularly useful in 5 situations:
Routine monitoring of water systems – If a water system is infected, then the faster this is identified, the faster corrective action can be taken. Many guidelines rely heavily on the risk assessment procedure, which requires that testing regimes are tailored to specific circumstances of the water system.
Checking systems where control measures have gone out of conformance – When cold water is not sufficiently cold, and hot water is not hot enough to prevent Legionella growth, or when biocide levels are too low, a system may become rapidly compromised. The real-time water safety checks can allow for quick identification of risk and instant remedial action.
Checking after a disinfection treatment – Once an infected water system has been treated, the faster it can be confirmed that the treatment has been effective, the faster everyone can get back to work. It also allows service providers to show their customer that the treatment has worked, offering them a peace of mind.
Identifying the source of an outbreak – If there is an outbreak it is important for both public health and for legal reasons to establish the source as soon as possible. The Hydrosense test can provide immediate detection and allows duty holders to take immediate remedial action. A positive with Hydrosense can ensure people are protected from a source of infection in minutes rather than continued exposure while water management professionals are waiting for Lab Culture or PCR results from a Lab.
In any environment without easy access to a lab – For many sites with critical water supplies, it is difficult, if not impossible, to send samples to a lab for testing. For example, on ships and offshore facilities like oil rigs, on-site testing provides easy and invaluable results that cannot be obtained from the lab.
Legionella testing frequency will depend on regulations in your industry and region, your company specific water safety plan and the level of risk facing your organisation/facility. For instance, hotels and medical facilities will have a much higher risk than office blocks because of more complex water systems and, in the case of medical facilities, the presence of more vulnerable people.
Samples for use with the Hydrosense kits can either be in the form of a water sample or a biofilm swab sample. Sampling sites for each of these should be chosen to be representative of all the identified risk areas where Legionella bacteria can reside and grow. It is important to remember that sampling procedure should follow a tailored Water Safety Plan that is specific to the organisation, country and industry. If you are unsure what areas should be tested in specific countries, please see recommendations below:
The UK – Legionella species: sampling of households
The UK – BS 7592:2022 Sampling for Legionella Bacteria in Water Systems
The EU – European Technical Guidelines for the Prevention, Control and Investigation, of Infections Caused by Legionella species
The Spanish Royal Decree aimed at the prevention and control of legionellosis
Australian enHealth Guidelines for Legionella Control in the Operation and Maintenance of Water Distribution Systems in Health and Aged Care Facilities
The USA – CDC, Sampling Procedure and Potential Sampling Sites
You can also visit our Legionella high-risk sectors page for more information.
When using the Hydrosense tests no particular protective equipment is required. The filtration step in some kits captures any bacteria in the sample thus reducing risk while taking the test. When collecting a water sample however, you should avoid creating aerosols (showers and spray). It is advised that you follow your Water Safety Plan recommendations for the collection of water samples for Legionella.
All of the equipment you need to carry out a test is included in the kit provided.
The tests should be stored at room temperature, preferably below 30°C (86°F). The tests can be exposed to slightly higher or lower temperatures for short periods of time without impacting the test, but should be returned to room temperature as soon as possible. The test should not be frozen. The tests are packaged in a waterproof foil wrapper, which must be undamaged. Damaged wrappers mean that the test may have been compromised and must not be used.
Used tests can be disposed of in normal waste procedures. The tests, even when exposed to Legionella, do not pose a risk of infection as sprays or aerosols cannot be created. On the instruction leaflet that you receive with each test kit, you will find a full list of components that can and cannot be recycled.
No. The tests are simple to use and no special training is required. Simply use the visual step-by-step instructions provided with each test kit.
A negative test result indicates that Legionella pneumophila sg1 and/or sg2-15 antigen (depending on which test you are using) has not been detected in your water system or that the concentration was below the limit of detection.
A positive Hydrosense Legionella test means that Legionella pneumophila sg1 and/or sg2-15 antigen has been detected in your water system above the limit of detection for the test kit used.
If Legionella has been detected in your water system, your staff, customers and the general public are at risk of contracting Legionnaires’ disease. This can have very serious consequences for you and your business. It is recommended to follow your Water Safety Plan recommendations, contact your water management specialist or clean your water system immediately.
The shelf life is 18 months from the date of manufacture when stored below 30°C. Tests are supplied with at least 12 months remaining on the shelf life. If you are concerned about the shelf life of the product, please discuss your requirements with a Hydrosense Team Member, before placing an order.
The Hydrosense range of tests are specific to the species Legionella pneumophila. The ONE range of tests detects L. pneumophila sg1, whilst the PRO range detects both L. pneumophila sg1 and L. pneumophila sg2-15. Legionella pneumophila serogroups 1-15 together are responsible for around 97% 1 of all global cases of Legionnaries’ disease with sg1 accounting for 90% 2 of the cases and the remainder caused by sg2-15. Other species of Legionella are much less common at causing human disease and therefore are not considered such high risk organisms.
The Hydrosense tests have all been rigorously tested and validated in house at Hydrosense to ensure the tests work exactly as specified. In addition, the Hydrosense test methods, both for ONE and PRO variations, have been independently validated by a UKAS accredited laboratory in the UK to verify the tests ability to detect Legionella pneumophila (sg1 and/or sg2-15) at the detection levels stated in our claims.
Both the Hydrosense ONE and PRO tests have also been validated by a CDC ELITE laboratory in the USA and the Hydrosense PRO test has been evaluated against lab culture using the CDC ELITE proficiency testing scheme and was found to be 100% accurate at identifying L. pneumophila from the blind samples used in testing scheme.
Many products sold in the EU carry a CE mark which indicates that the product is in compliance with European Directives. At present, there is no EU directive on any Legionella water tests. As a result, CE marking is not applicable.
UKAS Accreditation is only available to laboratories and therefore UKAS accreditation is not applicable to the Hydrosense tests. It is possible that a UKAS accredited lab could carry out Hydrosense Legionella rapid tests, but the tests are generally designed for on-site, rather than lab based testing.
MSDS are available for all of our Hydrosense test kits. To request an MSDS contact a member of the team at
Yes. Every batch of Hydrosense tests produced undergoes stringent QC testing to ensure it meets our specifications. A certificate of conformance can be obtained by contacting
Yes. The Hydrosense range of Legionella tests are highly efficient for testing multiple samples at one time and takes only 25 minutes to provide results. The filtration process typically takes well under 5 minutes. Several samples can be collected, and multiple different tests can be performed simultaneously.
Lab culture methods for Legionella testing, though widely recommended, have significant limitations. The ISO standard (11731:2017) indicates that lab culture recovery rates are often lower than 64%, meaning up to 35% of tests may return false negatives. Furthermore, lab culture fails to detect Viable but Non-Culturable (VBNC) forms of Legionella, which still pose significant infection risks.
Relying on lab culture alone may delay crucial action since results take up to two weeks — during this wait, people have been exposed to potential increased infection risks. Hydrosense, by contrast, delivers results in just 25 minutes. While lab tests are necessary for compliance in some cases, supplementing them with additional testing methods like Hydrosense can offer more immediate and reliable protection.
Legionnaires’ disease presents a significant risk to public health and as such, in many industries, the control of Legionella bacteria is carefully regulated by specific health and safety laws.
However, some businesses and sectors have a much higher risk when it comes to Legionella and Legionnaires’ disease than others. The main risk areas that encourage the rapid growth and spread of Legionella include cooling towers, potable water systems, showers, sinks, spas/hot tubs, swimming pools, water tanks, car washes, decorative fountains and others. Read more about risk factors in different sectors here.
Yes, The Hydrosense Legionella Tests have been developed to be very easy to conduct and with the help of the instructions included in the kit, and instructional videos available online, anyone can perform the test.
The Hydrosense test kits are particularly useful in 5 situations:
Routine monitoring of water systems – If a water system is infected, then the faster this is identified, the faster corrective action can be taken. Many guidelines rely heavily on the risk assessment procedure, which requires that testing regimes are tailored to specific circumstances of the water system.
Checking systems where control measures have gone out of conformance – When cold water is not sufficiently cold, and hot water is not hot enough to prevent Legionella growth, or when biocide levels are too low, a system may become rapidly compromised. The real-time water safety checks can allow for quick identification of risk and instant remedial action.
Checking after a disinfection treatment – Once an infected water system has been treated, the faster it can be confirmed that the treatment has been effective, the faster everyone can get back to work. It also allows service providers to show their customer that the treatment has worked, offering them a peace of mind.
Identifying the source of an outbreak – If there is an outbreak it is important for both public health and for legal reasons to establish the source as soon as possible. The Hydrosense test can provide immediate detection and allows duty holders to take immediate remedial action. A positive with Hydrosense can ensure people are protected from a source of infection in minutes rather than continued exposure while water management professionals are waiting for Lab Culture or PCR results from a Lab.
In any environment without easy access to a lab – For many sites with critical water supplies, it is difficult, if not impossible, to send samples to a lab for testing. For example, on ships and offshore facilities like oil rigs, on-site testing provides easy and invaluable results that cannot be obtained from the lab.
Legionella testing frequency will depend on regulations in your industry and region, your company specific water safety plan and the level of risk facing your organisation/facility. For instance, hotels and medical facilities will have a much higher risk than office blocks because of more complex water systems and, in the case of medical facilities, the presence of more vulnerable people.
Samples for use with the Hydrosense kits can either be in the form of a water sample or a biofilm swab sample. Sampling sites for each of these should be chosen to be representative of all the identified risk areas where Legionella bacteria can reside and grow. It is important to remember that sampling procedure should follow a tailored Water Safety Plan that is specific to the organisation, country and industry. If you are unsure what areas should be tested in specific countries, please see recommendations below:
The UK – Legionella species: sampling of households
The UK – BS 7592:2022 Sampling for Legionella Bacteria in Water Systems
The EU – European Technical Guidelines for the Prevention, Control and Investigation, of Infections Caused by Legionella species
The Spanish Royal Decree aimed at the prevention and control of legionellosis
Australian enHealth Guidelines for Legionella Control in the Operation and Maintenance of Water Distribution Systems in Health and Aged Care Facilities
The USA – CDC, Sampling Procedure and Potential Sampling Sites
You can also visit our Legionella high-risk sectors page for more information.
When using the Hydrosense tests no particular protective equipment is required. The filtration step in some kits captures any bacteria in the sample thus reducing risk while taking the test. When collecting a water sample however, you should avoid creating aerosols (showers and spray). It is advised that you follow your Water Safety Plan recommendations for the collection of water samples for Legionella.
All of the equipment you need to carry out a test is included in the kit provided.
The tests should be stored at room temperature, preferably below 30°C (86°F). The tests can be exposed to slightly higher or lower temperatures for short periods of time without impacting the test, but should be returned to room temperature as soon as possible. The test should not be frozen. The tests are packaged in a waterproof foil wrapper, which must be undamaged. Damaged wrappers mean that the test may have been compromised and must not be used.
Used tests can be disposed of in normal waste procedures. The tests, even when exposed to Legionella, do not pose a risk of infection as sprays or aerosols cannot be created. On the instruction leaflet that you receive with each test kit, you will find a full list of components that can and cannot be recycled.
No. The tests are simple to use and no special training is required. Simply use the visual step-by-step instructions provided with each test kit.
A negative test result indicates that Legionella pneumophila sg1 and/or sg2-15 antigen (depending on which test you are using) has not been detected in your water system or that the concentration was below the limit of detection.
A positive Hydrosense Legionella test means that Legionella pneumophila sg1 and/or sg2-15 antigen has been detected in your water system above the limit of detection for the test kit used.
If Legionella has been detected in your water system, your staff, customers and the general public are at risk of contracting Legionnaires’ disease. This can have very serious consequences for you and your business. It is recommended to follow your Water Safety Plan recommendations, contact your water management specialist or clean your water system immediately.
The shelf life is 18 months from the date of manufacture when stored below 30°C. Tests are supplied with at least 12 months remaining on the shelf life. If you are concerned about the shelf life of the product, please discuss your requirements with a Hydrosense Team Member, before placing an order.
Technical FAQ
The Hydrosense Legionella rapid tests use Lateral Flow Immunochromatographic Assay (LFICA) technology. This is the same technology as that used in pregnancy or COVID tests. Hydrosense tests are designed to detect Legionella antigens, using antibodies tagged with coloured nanoparticles, which bind to any appropriate Legionella bacteria found in a sample and make them visible as a line(s) on the test strip. For the ONE range of tests, the antidodies used are tagged with red nanoparticles which detect Legionella pneumophila sg1 bacteria, whilst the PRO range also uses antibodies tagged with green nanoparticles which detect Legionella pneumophila sg2-15 bacteria as well.
Legionella can change to a Viable But Non-Culturable (VBNC) state when exposed to environmental stresses such as biocide exposure, lack of nutrients, high temperature or UV treatment. In this state the bacteria will never be detected by lab culture.
Yes. VBNC bacteria can resuscitate when conditions are more suitable, often many months later. This ability to become dormant and reactivate is likely to be a reason why systems are very difficult to keep free of Legionella even when controls are in place. VBNC Legionella have also been shown to be able to directly infect human lung cells. As a result, VBNC bacteria should be treated as a risk for Legionnaires’ disease.
The Hydrosense tests detect Legionella pneumophila antigen. This means that the test is able to detect viable and Viable But Non-Culturable (VNBC) bacteria as well as non-viable cells.
Although the test is able to detect the antigen of non-viable cells in most cases this is unlikely as antigen tends to be removed from a system quickly or will be below detectable levels when there is no active Legionella growth occurring. If the test returns a positive result then action should be taken as the concentration of Legionella bacteria is very likely to be high enough to pose a significant risk to human health.
The Hydrosense tests measure levels of antigen in a sample and not Colony Forming Units (CFU) like lab culture does. There is however, a correlation between antigen quantity and number of cells – though it is worth noting that the number of antigen-containing cells could be higher than colony forming units detected from culture testing because VBNC cells may be present. A 3rd party Hydrosense study with PHE (Public Health England) Legionella lenticules of known levels of CFU has shown a linear relationship between the test signal and CFU counts – higher cell quantities show a stronger antigen response as indicated by a stronger test line. Some of our test kits are supplied with a hazard index card which gives a rough estimate of CFU based on the test signal of a positive test.

 Swedish
Swedish  Norwegian
Norwegian  Finnish
Finnish  English
English